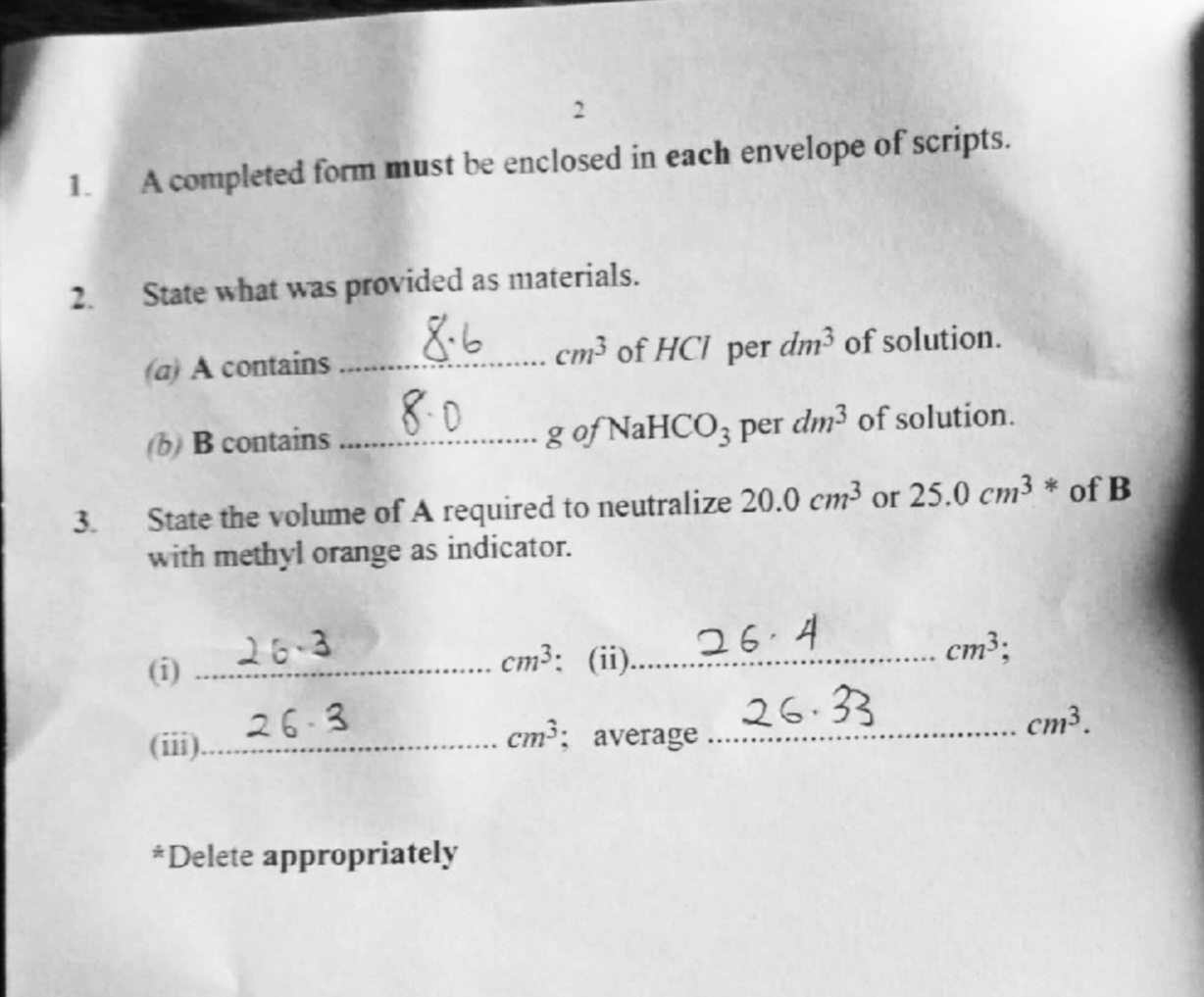
TEACHERS REPORT:
(2)
(a) 8.6
(b) 8.0
(3)
(i) 26.3
(ii) 26.4
(iii) 26. 3
Average 26.33
VERY IMPORTANT – PLEASE READ CAREFULLY
For Question 1:
We used 26.33cm³ as our own school’s end point.
You must use your own school’s end point instead.
Anywhere you see 26.33cm³ in our answer, change it to your own and calculate again.
Example:
In 1bii, we used 26.33cm³ to get CB = 0.0952
→ You should use your school’s end point to get your own CB.
Then in 1biii, we used our 0.0952 to calculate and got 8.0
→ You must use your own CB from 1bii to get your own answer in 1biii.
In 1biv, we used the 8.0 from 1biii to get our final percentage
→ You must use your own value from 1biii to get your correct answer in 1biv.
Chemistry students should understand this.
If confused, kindly ask your Chemistry teacher for help.
NOTE:
If your school wants to use our end point (26.33), the Chemistry teacher must agree and submit something close to it… like 26.10, 26.20, 26.30, etc.
Number 1
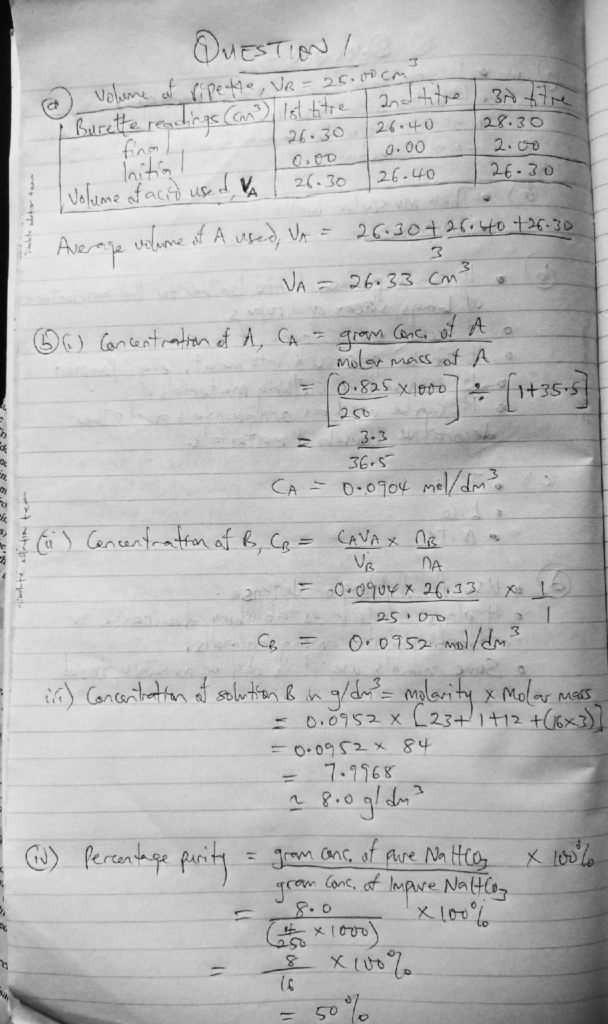
Number 2

Number 3
(3a)
Ammonia gas (NH₃).
(3b)
(PICK ANY ONE)
Dip a glass rod in concentrated hydrochloric acid and introduce it into the gas jar containing R; the formation of white fumes confirms the presence of ammonia gas.
OR
Expose the gas to hydrogen chloride (HCl) gas to observe formation of white fumes
OR
Introduce a glass rod soaked in concentrated HCl acid into the jar containing the gas; the appearance of dense white fumes confirms the presence of ammonia
(3c)
(NH₄)₂SO₄
(3d)
Ammonium tetraoxosulphate(VI)

Answers Loading………..









Neg
Kobozo
Exambot. net
hausa
Great job 👍